Answer:
'Low reactivity' is common property of noble gases.
Step-by-step explanation:
The properties of noble gases are:
- They are colorless, odorless and mono atomic gases.
- They are found in group number 18 of the modern periodic table.For example ; helium , xenon etc.
- They have completely filled
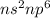 electronic configuration in their valence shells which accounts for their inert nature and low reactivity.
electronic configuration in their valence shells which accounts for their inert nature and low reactivity. - They have high ionization enthalpies.
- The electron gain enthalpies of noble gases are positive.