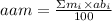Answer:
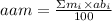
Step-by-step explanation:
When an atom has 2 or more isotopes, the average atomic mass (aam) depends on the mass of each isotope (mi) and the percentual abundance in nature of each isotope (abi). The average atomic mass can be calculated using the following expression: