Step-by-step explanation:
When we increase the temperature of a substance then there will occur an increase in the kinetic energy of its molecules.
Also, K.E =
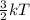
So, kinetic energy is directly proportional to the temperature.
Hence, when temperature and pressure are kept the same for both oxygen and hydrogen gas then values of their kinetic energy will be the same irrespective of their masses.
Thus, we can conclude that kinetic energy of oxygen molecule is the same as compared to hydrogen molecule.