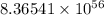Answer:
Step-by-step explanation:
Mass of the sum =
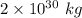
70% of this mass is
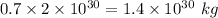
Mass of hydrogen in the sun is
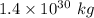
Mass of a hydrogen atom =
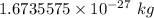
Dividing the mass hydrogen in the sun by the mass of one hydrogen atom will give us the number of hydrogen atoms
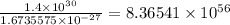
The number hydrogen atoms in the Sun is