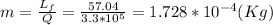Answer:
The mass of ice melted is:
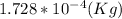
Step-by-step explanation:
We need to remember that the latent heat of fusion is related as:
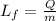 , where Lf is the latent heat of fusion, Q is the amount of heat and m is the mass, and when there is a change of phase, e.g ice into liquid water, the temperature remains constant during this process. Now we calculate the kinetic energy of the block as:
, where Lf is the latent heat of fusion, Q is the amount of heat and m is the mass, and when there is a change of phase, e.g ice into liquid water, the temperature remains constant during this process. Now we calculate the kinetic energy of the block as:
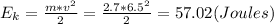 , so this energy is used to change ice into liquid water, and knowing
, so this energy is used to change ice into liquid water, and knowing
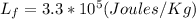 , then we can replace in the equation the latent heat of fusion and get the mass of ice melted as:
, then we can replace in the equation the latent heat of fusion and get the mass of ice melted as: