Answer:
Pressure predicted by van der waals equation is 1984.7 atm
Step-by-step explanation:
Van der waal gas equation-
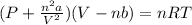
Where, P is pressure of gas , n is number of moles of gas, a is pressure correction constant, V is volume of gas, b is volume correction constant , R is gas constant and T is temperature in kelvin scale.
So, plug-in all the given values in the above equation-
![[P+((20.0mol)^(2)* (3.658L^(2).atm.mol^(-2)))/((1.0L)^(2))][1.0L-(20.0mol*0.04286 L.mol^(-1) )]=(20.0mol)* (0.08206L.atm.mol^(-1).K^(-1))* (300.0K)](https://img.qammunity.org/2020/formulas/chemistry/high-school/suv135iy25wgau6bb047luh5gn09cex3yf.png)
or,
![[P+((20.0mol)^(2)* (3.658L^(2).atm.mol^(-2)))/((1.0L)^(2))]](https://img.qammunity.org/2020/formulas/chemistry/high-school/fdj53mf5o89sg8oni3oihsbkumw1hnwpdf.png) =
=
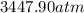
or, P = 1984.7 atm
So, Pressure predicted by van der waals equation is 1984.7 atm