Answer:
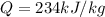
Step-by-step explanation:
Hello,
In this case, the transferred heat to the mixture is given by the addition among each compound's specific enthalpies:
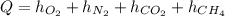
Now, each specific enthalpy is given by the following equation:
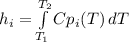
As long as there is a large difference between the initial and the final temperature. In such a way, the shown below polynomial for the Cp which is given kJ/kmol*K is considered and subsequently integrated:
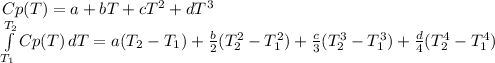
Now, the specific enthalpy is computed for each component considering the temperatures in Kelvin:
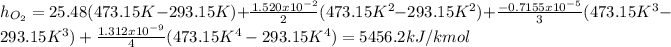
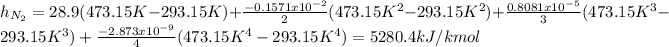
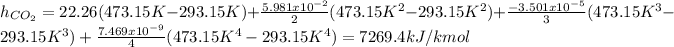
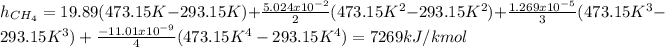
Now, we convert them per unit of mass as:
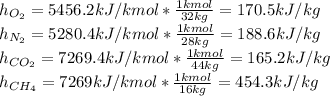
Finally, by considering the volumetric percentages, we compute the heat transferred to mixture:
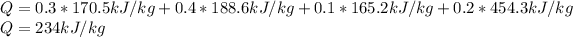
Best regards.