Answer:
6.(C) Magnesium and fluorine
7.(A) Oppositely charged ions attract
8.(C)
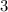
9.(d)
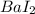
Step-by-step explanation:
6.An ionic bond is formed between a metal and a non metal.
Magnesium is a metal and fluorine is a non metal, so they form ionic bond.
7. Ionic compounds are electrically neutral as they consist of equal and opposite charges.
8. Nitrogen has
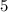 electrons in outermost orbit ,
electrons in outermost orbit ,
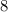 is required in the outermost orbit to gain the noble gas configuration.
is required in the outermost orbit to gain the noble gas configuration.
Thus,
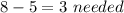
9. An ionic compound has a metal and a non metal, out of those four options only barium(Ba) is the metal combined with a non metal iodine(I)
Thus
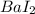 best fits.
best fits.
Thus those are the respective answers.