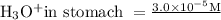
Step-by-step explanation:
Nexium (Generic name: Esomeprazole Magnesium) basically used to treat stomach acid problems, by decreasing amount of acid secretion and Increasing pH needed for body.
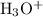 in stomach is gastric acid formed by following reaction
in stomach is gastric acid formed by following reaction
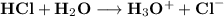
- The
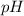 of a solution can be found by using formula:
of a solution can be found by using formula:
![-\log \left[\mathbf{H}_(3) \mathbf{O}^(+)\right]=p H](https://img.qammunity.org/2020/formulas/chemistry/middle-school/1rgfefppfl9yw27zn69de5v5ikfdfskgfn.png)
So here
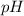 in Larry’s stomach = 4.52
in Larry’s stomach = 4.52
From above formula hydronium ion concentration is
![\left[\mathrm{H}_(3) \mathrm{O}^(+)\right]=10^(-p H)=10^(-4.52)=<strong>3.0 * 10^(-5) \mathrm{M}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/irfctwdmyvknddb2hsjce29v10v26hasa6.png)