Answer:
The formula for Lead(ll) Oxide is PbO.
Step-by-step explanation:
The symbol for Lead is Pb.
The symbol for Oxygen is O.
Given,Oxidation state of Pb = +2
We know,
Oxidation state of Oxygen in all Oxides is the same and is equal to -2,
Let the no. of Pb atoms in the oxide = x;
no.of Oxygen atoms = y;
Concept:
Sum of oxidation states of all atoms in any compound is equal to zero.
Applying concept,we get,
x(+2) + y(-2) = 0
x(+2) = y(+2)
Therefore, x = y
and, x : y = 1:1 ;
Therefore the empirical and the correct formula of the oxide is
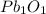 ,which is
,which is
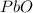 .
.