Answer:
More reactant forms.
Step-by-step explanation:
Given reaction is,
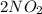 ⇒
⇒
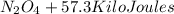 per mole
per mole
This is an Exothermic Reaction,(ΔE=-57.3KJper mole)
We know the equilibrium point of all Exothermic reactions moves leftward and more reactant is formed at the equilibrium.
Reason:
As heat is being produced in the reaction the additional heat(57.3KJpermole) can be thought of as a product of the reaction.
So,if you increase the temperature ,you provide heat energy,
(in other words heat energy is given) and hence the concentration of the products increases.
So, with respect to LeChateliers Principle,
As the concentration of products is increased by external means,more of the reactants are produced at the equilibrium of the reaction.
Therefore amount of reactants increases as more reactant forms.