Answer:
300.06 grams of glucose can be produced from a photosynthesis reaction that occurs using 10 moles of carbon dioxide.
Step-by-step explanation:
The balanced chemical reaction of photosynthesis will be:
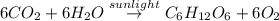
According to reaction, 6 moles of carbon dioxide gas gives 1 mole of glucose.
Then ,10 moles of carbon dioxide will gibve:
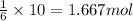 moles of glucose
moles of glucose
Mass of 1.667 moles glucose :
= 1.667 mol × 180 g/mol= 300.06 g
300.06 grams of glucose can be produced from a photosynthesis reaction that occurs using 10 moles of carbon dioxide.