Answer:
The solubility of MnS will decrease on addition of KOH solution.
Step-by-step explanation:
As per the equation given:

On dissolution of MnS in water it gives a basic solution as it gives hydroxide ions.
Now when the we are adding aqueous KOH solution, it will dissociate as:
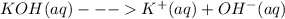
Thus it will further furnish more hydroxide ion,
This will increase the concentration of hydroxide ions (present of product side), the system will try to decrease its concentration by shifting towards reactant side.
Thus the solubility of MnS will decrease on addition of KOH solution.