Answer:
Answers are in the explanation.
Step-by-step explanation:
The law of multiple propotions says that "when two elements combine with each other to form more than one compound, the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers". Using this law it is possible to obtain empirical formula of the compounds, thus:
1. A, sample A.
0,330g Mn×
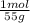 = 6x10⁻³ moles
= 6x10⁻³ moles
0,144g O×
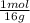 = 9x10⁻³ moles
= 9x10⁻³ moles
Ratio O:Mn = 9x10⁻³ / 6x10⁻³ = 1,5
To explain this ratio, empirical formula must be:
Mn₂O₃
1. A, sample B.
0,330g Mn×
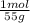 = 6x10⁻³ moles
= 6x10⁻³ moles
0,336g O×
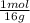 = 2,1x10⁻² moles
= 2,1x10⁻² moles
Ratio O:Mn = 2,1x10⁻² / 6x10⁻³ = 3,5
To explain this ratio, empirical formula must be:
Mn₂O₇
1. B, sample A.
0,508g I×
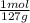 = 4x10⁻³ moles
= 4x10⁻³ moles
0,128g O×
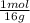 = 8x10⁻³ moles
= 8x10⁻³ moles
Ratio O:Mn = 8x10⁻³ / 4x10⁻³ = 2
To explain this ratio, empirical formula must be:
I₂O
1. B, sample B.
0,508g I×
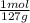 = 4x10⁻³ moles
= 4x10⁻³ moles
0,144g O×
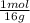 = 9x10⁻³ moles
= 9x10⁻³ moles
Ratio O:Mn = 9x10⁻³ / 4x10⁻³ = 2,25
To explain this ratio, empirical formula must be:
I₉O₄
2. Part A.
1,5L solution×
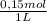 ×
×
 = 54,96g of BaCl₂.2H₂O
= 54,96g of BaCl₂.2H₂O
2. Part B.
2,5L solution×
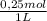 ×
×
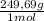 = 156,1g of CuSO₄.5H₂O
= 156,1g of CuSO₄.5H₂O
I hope it helps!