Step-by-step explanation:
We assume equal number of moles at constant temperature. Therefore,
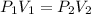
Hence,
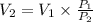
where,
 = atmospheric pressure = 1 atm
= atmospheric pressure = 1 atm
 = volume bag + volume gas left over in cylinder.
= volume bag + volume gas left over in cylinder.
Also we know that formula for volume of cylinder is as follows,
V =
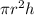
It is given here that, diameter of cylinder = 4.4 cm
Therefore, radius =
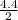
= 2.2 cm
and, h =17 cm
Hence, calculate value of
 as follows.
as follows.
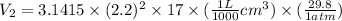
= 7.7 L
Thus, we can conclude that final volume of nitrogen gas, including what's collected in the plastic bag and what's left over in the cylinder is 7.7 L.