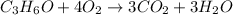Answer:
The empirical formula is =
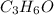
Balanced reaction is:
Step-by-step explanation:
Mass of water obtained = 2.51 g
Molar mass of water = 18 g/mol
Moles of
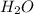 = 2.51 g /18 g/mol = 0.1394 moles
= 2.51 g /18 g/mol = 0.1394 moles
2 moles of hydrogen atoms are present in 1 mole of water. So,
Moles of H = 2 x 0.1394 = 0.2789 moles
Molar mass of H atom = 1.008 g/mol
Mass of H in molecule = 0.2789 x 1.008 = 0.2811 g
Mass of carbon dioxide obtained = 6.14 g
Molar mass of carbon dioxide = 44.01 g/mol
Moles of
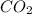 = 6.14 g /44.01 g/mol = 0.1395 moles
= 6.14 g /44.01 g/mol = 0.1395 moles
1 mole of carbon atoms are present in 1 mole of carbon dioxide. So,
Moles of C = 0.1395 moles
Molar mass of C atom = 12.0107 g/mol
Mass of C in molecule = 0.1395 x 12.0107 = 1.6755 g
Given that the compound only contains hydrogen, oxygen and carbon. So,
Mass of O in the sample = Total mass - Mass of C - Mass of H
Mass of the sample = 2.70 g
Mass of O in sample = 2.70 - 1.6755 - 0.2811 = 0.7434 g
Molar mass of O = 15.999 g/mol
Moles of O = 2.3028 / 15.999 = 0.0464 moles
Taking the simplest ratio for H, O and C as:
0.2789 : 0.0464 : 0.1395
= 6 : 1 : 3
The empirical formula is =
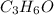
Balanced reaction is: