Answer:
a) 18,23 kgHCl/m³
b) 0,38 kg HCl/s
c) 0,018 kgHCl/kgsolution
d) 8,75x10⁻³ molHCl/molH₂O
e) 0,010 kmolHCl/s
Step-by-step explanation:
The HCl solution is 0,50 mol/L; it flows in a rate of 1,25 m³/min; Its density is 1,03 kg/L. HCl weights 36,46 g/mol
a) The HCl concentration in kg/m³ is:
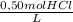 ×
×
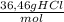 ×
×
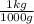 ×
×
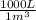 = 18,23 kg HCl/m³
= 18,23 kg HCl/m³
b) As you have a concentration of 18,23 kgHCl/m³
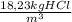 ×
×
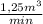 ×
×
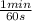 = 0,38 kg HCl/s
= 0,38 kg HCl/s
c) The mass fraction in kgHCl/kgsolution is:
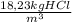 ×
×
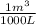 ×
×
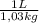 = 0,018 kgHCl/kgsolution
= 0,018 kgHCl/kgsolution
d) Supposing L of solution are just of water:
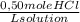 ×
×
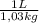 ×
×
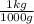 ×
×
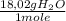 = 8,75x10⁻³ molHCl/molH₂O
= 8,75x10⁻³ molHCl/molH₂O
e) As the flow is 0,38kg HCl/s:
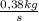 ×
×
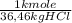 = 0,010 kmolHCl/s
= 0,010 kmolHCl/s
I hope it helps!