The emission spectrum of an element may be identical to the absorption spectrum of another element.
Answer: Option C
Explanation:
The emission spectrum acts as fingerprint of each element. As each element consists of discrete energy levels, so does the emission spectrum. As emission spectrum occurs when electron emit from higher energy orbitals to lower energy orbitals releasing an electromagnetic radiations in the form of loss of energy.
And absorption spectrum of an element is obtained when the element absorbs a specific wavelength of light incident to it. So, in absorption spectrum, the wavelengths which are absorbed by element will show a black line in its position.
Thus, emission spectrum obtained for an element is inversely related to the absorbance spectrum of the same element. If we represent it mathematically, it will look like ,
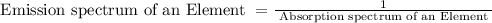
So, if we consider two different elements
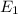 and
and
 , then
, then
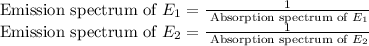
Now if we take ratio of emission spectrums, we will get
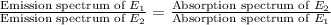
So, according to this, we can say that emission spectrum of an element can be identical to absorption spectrum of any other element.