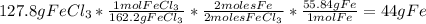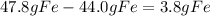Answer:
(a) The limiting reagent is the

(b)
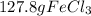 are formed
are formed
(c)
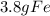 remain after the reaction is complete
remain after the reaction is complete
Step-by-step explanation:
First we are going to write down the balanced equation:
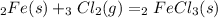
Then we are going to find the number of moles of each reactant:
-For Fe:
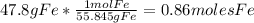
-For
 :
:
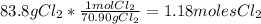
Then we are going to divide the number of moles by the stoichiometric coefficient for each reactant to find the limiting reagent:
-For Fe:
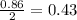
-For
 :
:
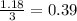
So, the limiting reagent is the
 .
.
Then we are going to find the maximum amount of iron(III) chloride that can be formed, so we take the limiting reagent for the calculations:
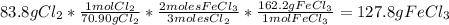
Finally we are going to calculate the amount of the excess reagent that remains after the reaction is complete: