Answer:
D Ionic bond
Step-by-step explanation:
Atomic number = number of electrons = number of protons.
Atomic number of sodium is 11
So the atom contains 11 protons and 11 electrons
To find the number of neutrons we make use of the formula
Mass number - atomic number = number of neutrons
From the periodic table we know mass number of sodium is 23
So number of neutron = 23 - 11 = 12.
When a sodium atom loses an electron it will have 11 positive protons and 10 negative electrons. Since 1 positive charge is more Na becomes
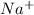 .
.
Positively charged ion is called as cation
Bromine atomic number is 35 so it has 35 protons and 35 electrons.
When it gains an electrons it will have 35 positive protons and 36 negative electrons since 1 negative charge is more Br becomes
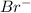 .
.
Negatively charged ion is called as anion.
The electrostatic attraction between
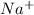 and
and
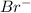 due to the opposite charges gets attracted and forms a bond called ionic bond in NaBr
due to the opposite charges gets attracted and forms a bond called ionic bond in NaBr
Look at the diagram of NaCl for example