Step-by-step explanation:
The given reaction will be as follows.
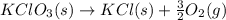
Molar mass of
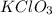 = 122.5 g/mol
= 122.5 g/mol
Molar mass of KCl = 74.5 g/mole
Molar mass of
 = 32 g/mole
= 32 g/mole
We assume that the mass of
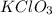 in the sample be x g
in the sample be x g
Therefore, the mass of KCl in the sample = 0.95 - x
Hence, moles of
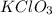 =
=
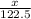
So, moles of KCl produced = moles of
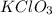 =
=
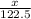
or, mass of KCl produced =
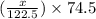
Therefore, total mass of KCl = mass of residue = (0.95 - x) +
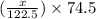 = 0.820 g
= 0.820 g
-0.39x = 0.820 - 0.95
x = 0.33
Thus, % of
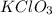 in the original sample will be calculated as follows.
in the original sample will be calculated as follows.
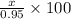
= 34.73%
Thus, we can conclude that mass % of
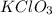 is 34.73%.
is 34.73%.