Answer:
Heat of vaporization will be 22.59 j
Step-by-step explanation:
We have given mass m = 10 gram
And heat of vaporization L = 2.259 J/gram
We have to find the heat required to vaporize 10 gram mass
We know that heat of vaporization is given by
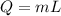 , here m is mass and L is latent heat of vaporization.
, here m is mass and L is latent heat of vaporization.
So heat of vaporization Q will be = 10×2.259 = 22.59 J