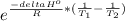Answer:
A. for K>>1 you can say that the reaction is nearly irreversible so the forward direction is favored. (Products formation)
B. When the temperature rises the equilibrium is going to change but to know how is going to change you have to take into account the kind of reaction. For endothermic reactions (the reverse reaction is favored) and for exothermic reactions (the forward reaction is favored)
Step-by-step explanation:
A. The equilibrium constant K is defined as
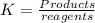
In any case
aA +Bb equilibrium Cd +dD
where K is:
![K= ([C]^(c)[D]^(d))/([A]^(a)[B]^(b))](https://img.qammunity.org/2020/formulas/chemistry/high-school/q5ro64lf67pqoilnu4lwuifmzz8ldz8pp3.png)
[] is molar concentration.
If K>>> 1 it means that the molar concentration of products is a lot bigger that the molar concentration of reagents, so the forward reaction is favored.
B. The relation between K and temperature is given by the Van't Hoff equation
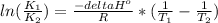
Where: H is reaction enthalpy, R is the gas constant and T temperature.
Clearing the equation for
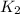 we get:
we get:
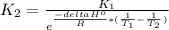
Here we can study two cases: when delta
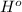 is positive (exothermic reactions) and when is negative (endothermic reactions)
is positive (exothermic reactions) and when is negative (endothermic reactions)
For exothermic reactions when we increase the temperature the denominator in the equation would have a negative exponent so
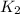 is greater that
is greater that
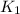 and the forward reaction is favored.
and the forward reaction is favored.
When we have an endothermic reaction we will have a positive exponent so
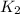 will be less than
will be less than
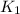 the forward reactions is not favored.
the forward reactions is not favored.