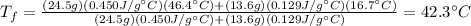Answer:
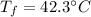
Step-by-step explanation:
The heat released by the iron is absorbed by the gold. This quantity is calculated with the formula
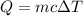 , which tells us the amount of heat (energy) Q needed to increase the temperature of a material of mass m, specific heat c by a temperature difference
, which tells us the amount of heat (energy) Q needed to increase the temperature of a material of mass m, specific heat c by a temperature difference
 .
.
Since the heat released (which we will take as negative Q) by the iron (Fe) is absorbed (positive Q then) by the gold (Au) we write
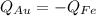 , which means
, which means
 , which is to say
, which is to say
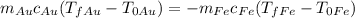 .
.
We know that thermal equilibrium has been reached, which means that at the end everything has the same temperature, so we have
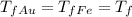 , and we can substitute
, and we can substitute
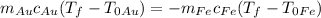 and do
and do
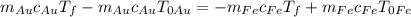 , to get finally
, to get finally
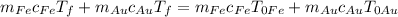 , which means
, which means
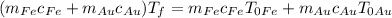 , giving us the final formula
, giving us the final formula
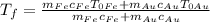 .
.
We can get from tables that
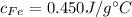 and
and
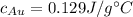 , so we put everything in a calculator and we get
, so we put everything in a calculator and we get