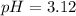Answer:
pH = 3.12
Step-by-step explanation:
To solve the problem you need to know that the pH is equal to the logarithm of the concentration of hydrogen ions, with negative sign. Or in other words pH is defined by:
![pH=-log[H^(+)]](https://img.qammunity.org/2020/formulas/medicine/college/11uazg5asji4j9glisfdrlkgsy6l390iy3.png)
As the problem gives you the concentration of hydrogen ions, you should replace the value:
![pH=-log[7.5*10^(-4)]](https://img.qammunity.org/2020/formulas/chemistry/college/jtb2zk7rpa890584tr0nc6t682rlbik0hk.png)