Answer: The volume of hydrogen gas that will be collected is 1.85 L
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of aluminium = 1.35 g
Molar mass of aluminium = 27 g/mol
Putting values in above equation, we get:
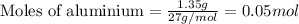
For the given chemical reaction:
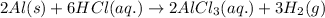
As, hydrochloric acid s present in excess. So, it is considered as an excess reagent.
Thus, aluminium is a limiting reagent because it limits the formation of products.
By Stoichiometry of the reaction:
2 moles of aluminium produces 3 moles of hydrogen gas
So, 0.005 moles of aluminium will produce =
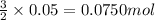 of hydrogen gas
of hydrogen gas
To calculate the mass of helium gas, we use the equation given by ideal gas:
PV = nRT
where,
P = Pressure of hydrogen gas = 743 Torr
V = Volume of the helium gas = ?
n = number of moles of hydrogen gas = 0.075 mol
R = Gas constant =
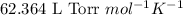
T = Temperature of hydrogen gas =
![21^oC=[21+273]K=294K](https://img.qammunity.org/2020/formulas/chemistry/high-school/7h54nhyoxlewxbmcja3skd50q1of2tc2dc.png)
Putting values in above equation, we get:
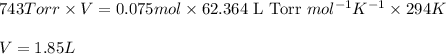
Hence, the volume of hydrogen gas that will be collected is 1.85 L