Answer: Option (a) is the correct answer.
Step-by-step explanation:
A chemical change is defined as the change which brings difference in the composition of reacting species.
Therefore, during a chemical change there will always be formation of new compounds.
For example,
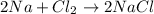 is a chemical change as new substance formed is NaCl.
is a chemical change as new substance formed is NaCl.
So basically, a chemical reaction equation or system tells the overall change occuring in the system.
Mechanism of a reaction can only be determined theoretically and not just by examining a chemical system.
Intermediates cannot be isolated as they are very reactive species. Hence, they cannot be determined by examining a chemical system.
Activated complex are the intermediate substances which are formed during the reaction and they cannot be isolated. Hence, they cannot be determined by examining a chemical system.
Thus, we can conclude that examining a chemical system before and after a reaction reveals the net chemical change.