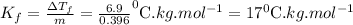Answer:
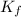 for solvent is
for solvent is
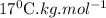
Step-by-step explanation:
- Let's assume that the solute is non-volatile as well as non-electrolyte.
- For a solution with non-volatile solute and non-electrolyte solute-
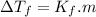 , where
, where
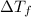 is depression in freezing point and m is molality of solution
is depression in freezing point and m is molality of solution
Molality of solution (m) = (moles of solute/mass of solvent in kg)
=
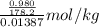
= 0.396 mol/kg
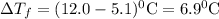
So,