Answer:
20 mg/ml.
Explanation:
We have been given that a liquid oral concentrate of morphine sulfate contains 2.4 g of morphine sulfate in a 120-mL bottle.
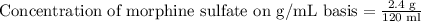
To convert the concentration of morphine sulfate on a mg/mL basis, we need to convert 2.4 grams to milligrams.
1 gram equals 1000 milligrams.
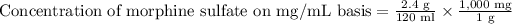
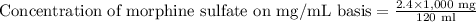
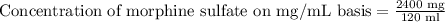
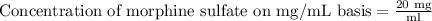
Therefore, the concentration of morphine sulfate would be 20 mg per ml.