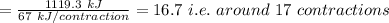Answer:
Number of contraction cycles that could theoretically be fueled by the complete combustion of one mole of glucose is around 17
Step-by-step explanation:
Energy released during the complete combustion of 1 mole glucose = 2870 kJ
Energy required/muscle contraction cycle = 67 kJ/contraction
Energy conversion efficiency = 39%
Actual amount of energy converted to contraction per mole of glucose is:

Total contraction cycles fueled by the above energy is: