Answer:
Option A
Step-by-step explanation:
Hi, this is why A is the correct option:
Hydronium ion (
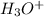 ), water (
), water (
 ) and hydroxide ion (
) and hydroxide ion (
 ) are all molecules formed by two or more atoms.
) are all molecules formed by two or more atoms.
So the correct answer is between hydrogen ion and electron. Well the diference is that the first one is positively charged (as the atom pointed) and the second one is negatively charged.
By elimination of the other options, the right one is option A