Answer : The normality of the solution is, 30.006 N
Explanation :
Normality : It is defined as the number of gram equivalent of solute present in one liter of the solution.
Mathematical expression of normality is:
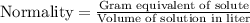
or,
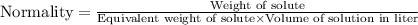
First we have to calculate the equivalent weight of solute.
Molar mass of solute
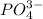 = 94.97 g/mole
= 94.97 g/mole
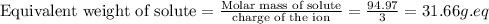
Now we have to calculate the normality of solution.
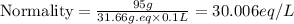
Therefore, the normality of the solution is, 30.006 N