Answer:
(b) Bronsted base
Step-by-step explanation:
The hydroxide ion is formed when
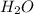 losses
losses
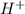 ion
ion
We know hat the compound which losses
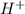 is known as the bronsted acid and the ion which is formed after losing
is known as the bronsted acid and the ion which is formed after losing
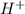 is known as bronsted base
is known as bronsted base
As the hydroxide ion is formed after losing the hydrogen ion from
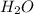 so it is a bronsted base.
so it is a bronsted base.