Answer : The number of moles of Sn(s) oxidized are 12 moles.
Explanation :
The given half cell reactions are:
Reduction half reaction :
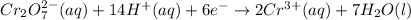
Oxidation half reaction :
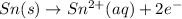
In order to balance the electrons, we will multiply oxidation half reaction by 3, we get:
Reduction half reaction :
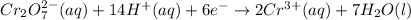
Oxidation half reaction :
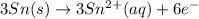
Now adding both half reaction, we get the overall reaction.
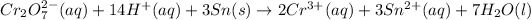
From the overall complete reaction, we conclude that
As, 1 mole of
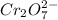 will oxidizes 3 moles of Sn
will oxidizes 3 moles of Sn
So, 4 moles of
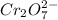 will oxidizes
will oxidizes
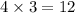 moles of Sn
moles of Sn
Therefore, the number of moles of Sn(s) oxidized are 12 moles.