Answer:
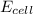 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous
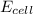 = -ve, reaction is non spontaneous
= -ve, reaction is non spontaneous
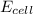 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
Case 1: when copper metal is combined with aqueous zinc sulfate.
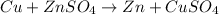
Here copper is undergoing oxidation ad thus acts as anode and zinc is undergoing reduction , thus acts as cathode.
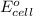 = standard electrode potential =
= standard electrode potential =
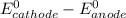
Where both
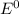 are standard reduction potentials.
are standard reduction potentials.
![E^0_([Cu^(2+)/Cu])= +0.34V](https://img.qammunity.org/2020/formulas/chemistry/college/jtti6ijw7m7t2w9u1bg1kxb4yfv5m7jft2.png)
![E^0_([Zn^(2+)/Zn])= -0.76V](https://img.qammunity.org/2020/formulas/chemistry/college/92kagvev0ox41l5zf6f3hrus121uooywpn.png)
![E^0=E^0_([Zn^(2+)/Zn])- E^0_([Cu^(2+)/Cu])](https://img.qammunity.org/2020/formulas/chemistry/college/jnnuydzinud53nlmwr6wese504weeojrwz.png)
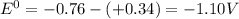
Thus as
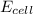 is negative , the reaction is non spontaneous.
is negative , the reaction is non spontaneous.
Case 2: when zinc metal and aqueous copper sulfate solution are combined.
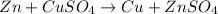
Here zinc is undergoing oxidation ad thus acts as anode and copper is undergoing reduction , thus acts as cathode.
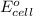 = standard electrode potential =
= standard electrode potential =
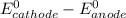
Where both
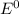 are standard reduction potentials.
are standard reduction potentials.
![E^0_([Cu^(2+)/Cu])= +0.34V](https://img.qammunity.org/2020/formulas/chemistry/college/jtti6ijw7m7t2w9u1bg1kxb4yfv5m7jft2.png)
![E^0_([Zn^(2+)/Zn])= -0.76V](https://img.qammunity.org/2020/formulas/chemistry/college/92kagvev0ox41l5zf6f3hrus121uooywpn.png)
![E^0=E^0_([Cu^(2+)/Cu])- E^0_([Zn^(2+)/Zn])](https://img.qammunity.org/2020/formulas/chemistry/college/xybit5pxhzd04ydj3m9ogn3rrqbglusezi.png)
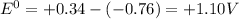
Thus as
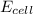 is positive , the reaction is spontaneous.
is positive , the reaction is spontaneous.