Answer:
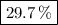
Step-by-step explanation:
We will need an equation with molar masses, so let’s gather all the information in one place.
M_r: 84.31 44.01
MgCO₃ ⟶ MgO + CO₂
The mass lost was that of CO₂.
1. Mass of CO₂
Mass of CO₂ = 20.69 g - 17.48 g = 3.21 g CO₂
2. Moles of CO₂
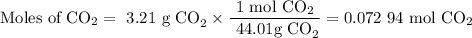
3. Moles of MgCO₃
The molar ratio is 1 mol MgCO₃:1 mol CO₂
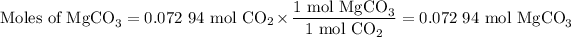
4. Mass of MgCO₃
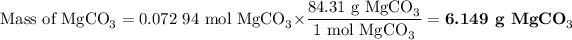
5. % MgCO₃
