Answer: The percent strength of the cell is 22.03 % and the enema is hypertonic in nature.
Step-by-step explanation:
Isotonic solutions are defined as the solutions in which concentration of solute inside and outside the cell is same.
Hypotonic solutions are defined as the solutions in which concentration of solute inside the cell is less than outside the cell.
Hypertonic solutions are defined as the solutions in which concentration of solute inside the cell is more than outside the cell.
Isotonic saline solution of normal cell in a human body is 0.9 % NaCl.
- To calculate the percentage strength of enema, we use the equation:
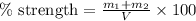
where,
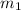 = mass of one product (monobasic sodium phosphate) = 19 g
= mass of one product (monobasic sodium phosphate) = 19 g
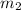 = mass of another product (dibasic sodium phosphate) = 7 g
= mass of another product (dibasic sodium phosphate) = 7 g
V = volume of solution = 118 mL
Putting values in above equation, we get:
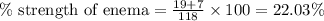
As, the concentration of enema inside the cell is 22.03 % which is way too high than the normal concentration of cell in human body. Thus, enema is hypertonic in nature.
Hence, the percent strength of the cell is 22.03 % and the enema is hypertonic in nature.