Answer:
Yes, with change in humidity, enthalpy changes as well.
Step-by-step explanation:
This can be explained by the given relation:
H =
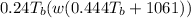
where
H = enthalpy (in Btu/lb)
w = specific humidity (lb-water/lb-dry air)
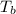 = dry-bulb temperature (in
= dry-bulb temperature (in
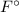 )
)
The above relation clearly explains that if temperature does not vary, enthalpy is in direct proportion with the humidity.
So, any change in humidity results in a corresponding change in the enthalpy.