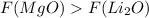Answer:
Magnesium oxide has stronger ionic attraction
Step-by-step explanation:
Ionic attraction force (F) is represented as-
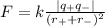
where k is constant,
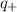 is charge on cation,
is charge on cation,
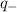 is charge on anion,
is charge on anion,
 is radius of cation and
is radius of cation and
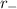 is radius of anion.
is radius of anion.
Here anion for both oxides is
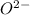 . So, for both of them
. So, for both of them
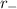 is same.
is same.
For lithium oxide
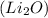 , cation is
, cation is
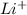 . So,
. So,
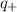 is +1.
is +1.
For magnesium oxide (MgO), cation is
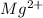 . So
. So
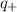 is +2
is +2
Hence
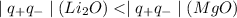 .
.
As, radius of lithium ion is greater than radius of magnesium ion therefore-

So altogether