Answer:
pH = pKa = 4.75
Step-by-step explanation:
The pH of a buffer solution is calculated using Henderson Hassalbalch's equation.
The equation is:
![pH=pKa+log([salt])/([acid])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xlklcv7b1496sz2ozqct518piznfdmytqu.png)
Where
pKa = -log (Ka) = 4.75 [for acetic acid]
[salt] = concentration of salt or the conjugate base, in the given example it is sodium acetate
[acid] = concentration of acid , here it is acetic acid
As given that the concentration of both the salt and acid is same it means
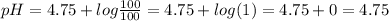
Thus the pH of solution will be equal to its pKa.