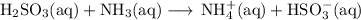Answer:
Here's what I get.
Step-by-step explanation:
A. Molecular equation
Sulfurous acid and ammonia are each weak electrolytes, so we write their formulas as molecules.
They undergo an acid/base reaction to form the soluble salt ammonium hydrogensulfite.
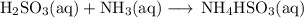
B. Ionic equation
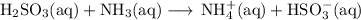
C. Net ionic equation
To get the net ionic equation, we cancel the ions that appear on each side of the ionic equation.
In this case, there are no ions to cancel, so the net ionic equation is the same as the ionic equation: