Answer:
0.9715 Fraction of Pu-239 will be remain after 1000 years.
Step-by-step explanation:
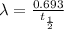
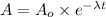
Where:
 = decay constant
= decay constant
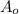 =concentration left after time t
=concentration left after time t
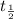 = Half life of the sample
= Half life of the sample
Half life of Pu-239 =
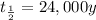 [
[
![\lambda =(0.693)/(24,000 y)=2.8875* 10^(-5) y^{-1]](https://img.qammunity.org/2020/formulas/chemistry/college/byobra2nqvjxb6gwqwnt0fnepb34v046he.png)
Let us say amount present of Pu-239 today =
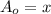
A = ?
![A=x* e^{-2.8875* 10^(-5) y^(-1]* 1000 y)](https://img.qammunity.org/2020/formulas/chemistry/college/tbelocx7wiy5z5kvg3os7m4sg9ar0i616j.png)
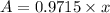
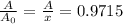
0.9715 Fraction of Pu-239 will be remain after 1000 years.