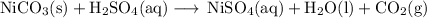Answer:
Here's what I get
Step-by-step explanation:
1. Nickel sulfate
base + acid ⟶ salt + water
NiSO₄ is a salt of the base Ni(OH)₂ and the acid sulfuric acid.
Hydroxides of transition metals are insoluble; most sulfates are soluble.
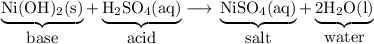
2. Carbonate + acid
Most carbonates are insoluble.
They react with acids to form carbonic acid (H₂CO₃), which decomposes into water and carbon dioxide.