Answer:
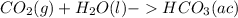 ; The pH decrease.
; The pH decrease.
Step-by-step explanation:
In general, when a nonmetal oxide reacts with water, the reaction produces an acid (that what is in the equation). Nevertheless, when the acid is produced, this acid gets dissolved by this process:
HCO_3 (ac) -> H^+ + (CO3)^-2\\[/tex]
This means that the concentration oh H^+ and (CO3)^-2, increase in the ocean water. The concentration of the H+ is directly related to the pH as the equation for the pH is pH=-log[H+].
As we have a negative sign in the logarithmic operation the relationship between the pH and the H+ is inversely proportional, in this way if we have more H+, the pH decrease.