Given:
Propylene is
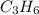
Degree of polymerization,
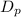 = 25000
= 25000
Solution:
no. of carbon atoms,
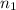 = 3
= 3
no. of hydrogen atoms,
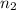 = 6
= 6
atomic weight of carbon,
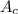 =12.01 g/mol
=12.01 g/mol
atomic weight of hydrogen,
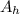 = 1.008 g/mol
= 1.008 g/mol
molecular mass of propylene, m =
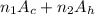
m =
 g/mol
g/mol
Now,
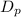 =
=
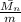
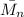 = m(
= m(
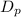 )
)
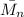 =
=
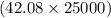
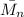 = 1052000 g/mol
= 1052000 g/mol
where,
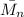 = number-average molecular weight
= number-average molecular weight