Answer:

Step-by-step explanation:
As per de Broglie theory we know that it is given as

now here we can say that by the principle of uncertainty we have
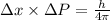
now we can use it to find the uncertainty in position as

now plug in the value of momentum as per de Broglie theory


So above is the maximum uncertainty in position in terms of de Broglie wavelength