Answer:
That's called a first-order reaction.
Step-by-step explanation:
In kinematics, the order of a chemical reaction is the sum of the power of concentrations in is rate law.
For example, consider a reaction with the following rate law:
![\text{Rate} = k\cdot [\mathrm{A}]^(a)\cdot [\mathrm{B}]^(b)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/81rqkixkj0hnrm4fu6b18zg5p4hjvkxqls.png) ,
,
where
![[\mathrm{A}]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/yodt6hgprdh164kpjjpumx09vfqikxvm8m.png) and
and
![[\mathrm{B}]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/9svkmt25ku39l833eyttx6j0rr2spfglrv.png) are the concentrations of the two reactants,
are the concentrations of the two reactants,
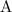 and
and
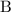 .
.
The order of this reaction will be equal to the sum of the powers of the concentrations in the rate law. For this sample reaction, the order is equal to
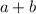 .
.
For the reaction in this question, the rate law will resemble the following:
![\text{Rate} = k\cdot [\mathrm{A}]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/5s5mzpuqwpk41es61nsn2gshbjcm2tgfrv.png) .
.
Note that the power "
 " next to the concentration of
" next to the concentration of
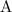 is omitted. The order of this reaction will be numerically equal to one.
is omitted. The order of this reaction will be numerically equal to one.
However, by convention, the order of the reaction is named in ordinals. (That is: first, second, third, etc.) The reaction here is known as a first-order reaction.
(Reference: "The Rate Law", Physical & Theoretical Chemistry, Chemistry Libretexts; "Cardinal and Ordinal Numbers Chart", Math Is Fun)