Answer:
3.85L
Step-by-step explanation:
Given parameters:
Initial pressure P₁ = 15psi (1psi = 52mmHg)
converting to mmHg gives (15x52)mmHg = 780mmHg
Initial volume V₁ = 7.0L
Final pressure P₂ = 1420torr= 1420mmHg
Unknown:
Final volume V₂ = ?
Condition of the process: Constant temperature
Solution
To solve this problem, we simply apply Boyle's law. Boyle's law states that "The volume of a given mass of gas varies inversely as the pressure changes, if the temperature is constant".
It is mathematically expressed as ;
P₁V₁ = P₂V₂
The unknown here is V₂ and we simply express it as the subject of the formula:
V₂ =
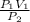
V₂ =
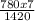
V₂ =
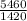 = 3.85L
= 3.85L