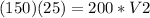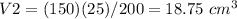Answer:
Option D. 18.75 cm3
Explanation:
we know that
The Boyle's law (Ideal gas law) states that Where temperature remains constant, the pressure is inversely proportional to volume.
so
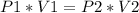
In this problem we have
P1=150 kPa
P2=200 kPa
V1=25 cm³
substitute and solve for V2