Answer : A real gas behave ideally at high temperature and low pressure condition.
Explanation :
The conditions for ideal gas are :
Ideal gas are those gas that has no intermolecular attractions.
Ideal gas are those gas that have negligible volume.
The ideal gas equation is,

The conditions for real gas are :
Real gas are those gas that have intermolecular attractions.
Real gas are those gas that have volume.
The real gas equation is,
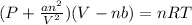
Thus, A real gas behave ideally at high temperature and low pressure condition.